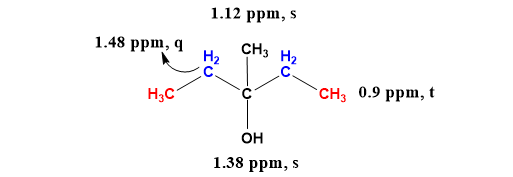
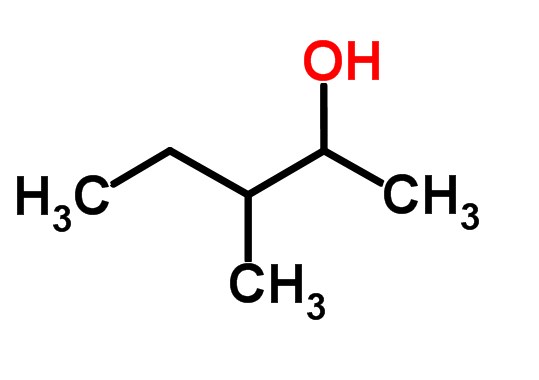
SOLVED: Spccify both the alcohol starting ' matcrial and the reagents you would use in cach stcp in a synthesis of the compound shown: If the synthesis requires only two steps enter
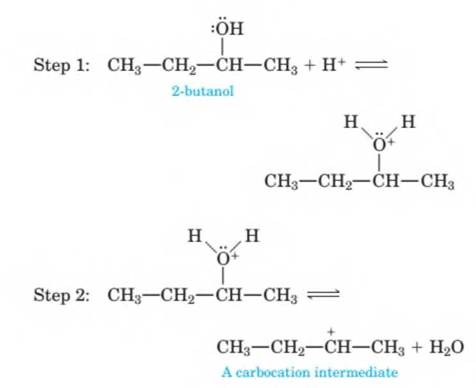
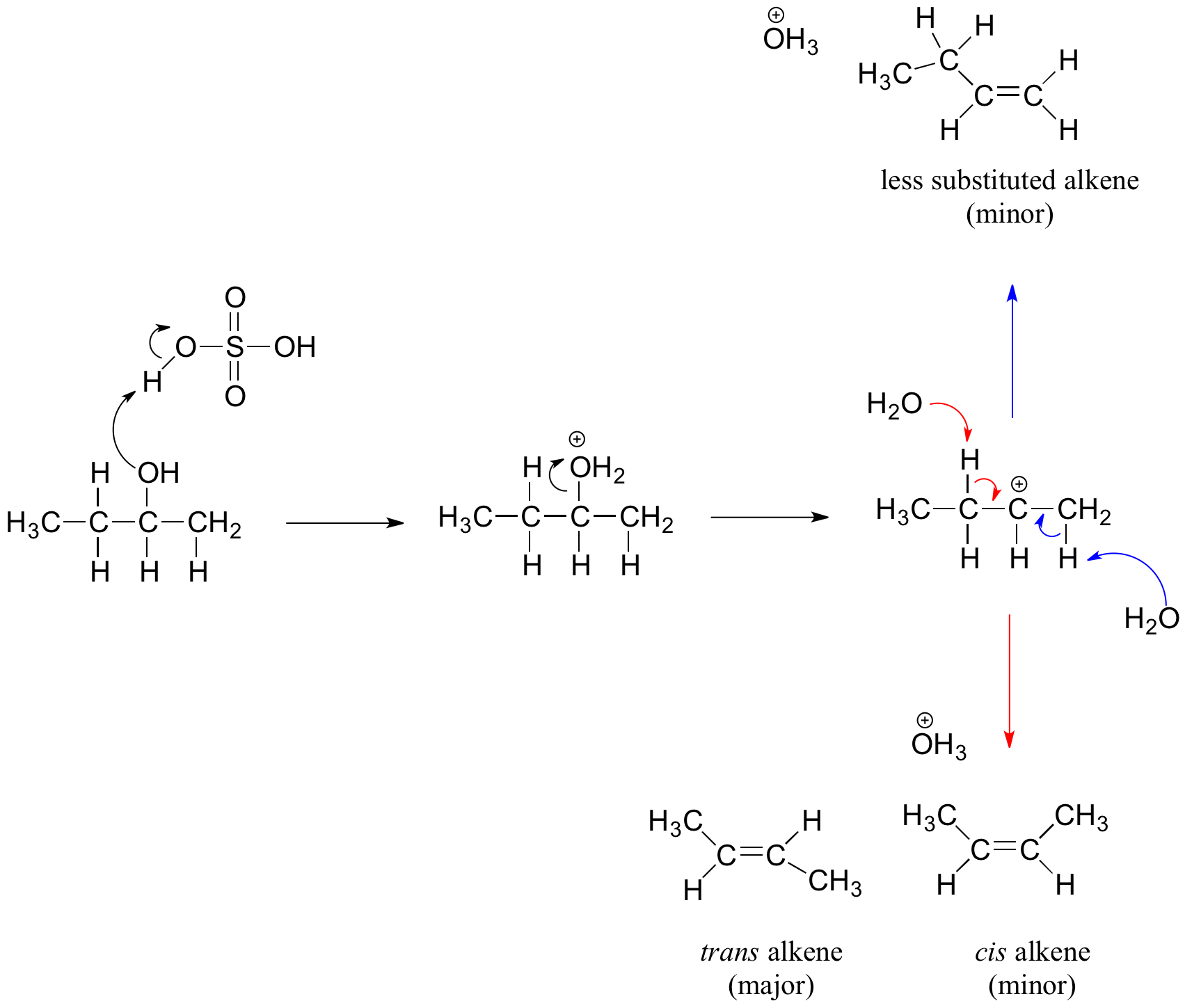
14-71 The mechanism of the acid-catalyzed dehydration of an alcohol to an alkene is the reverse of the acid- catalyzed hydration of an alkene. The dehydration mechanism occurs by the following three

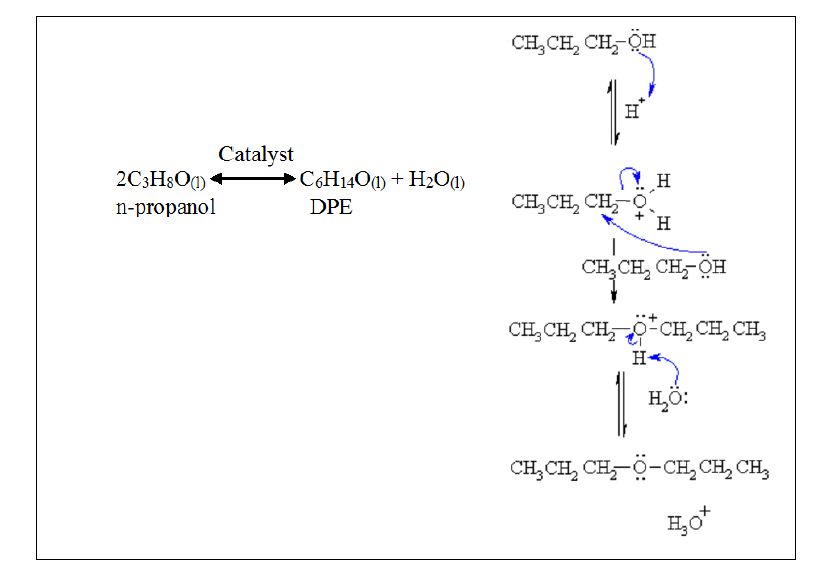
Dehydration of Pinacolyl Alcohol - Dehydration of Pinacolyl Alcohol Introduction H2SO4 H2SO4 C6H14O C6H12 C6H12 Figure 1. Chemical Equation for the | Course Hero

SOLVED: Draw the reactants and products for each of the chemical rcactions below: Dehydration of pure hexanol at 180*€ in the presence of H,SOs Dehydration of a mixture ethanol and [-propanol at

Saline-Sodium Citrate (SSC), 20X Solution (Molecular Biology), Fisher BioReagents Nalgene Poly Bottle; 1L Saline-Sodium Citrate (SSC), 20X Solution (Molecular Biology), Fisher BioReagents | Fisher Scientific
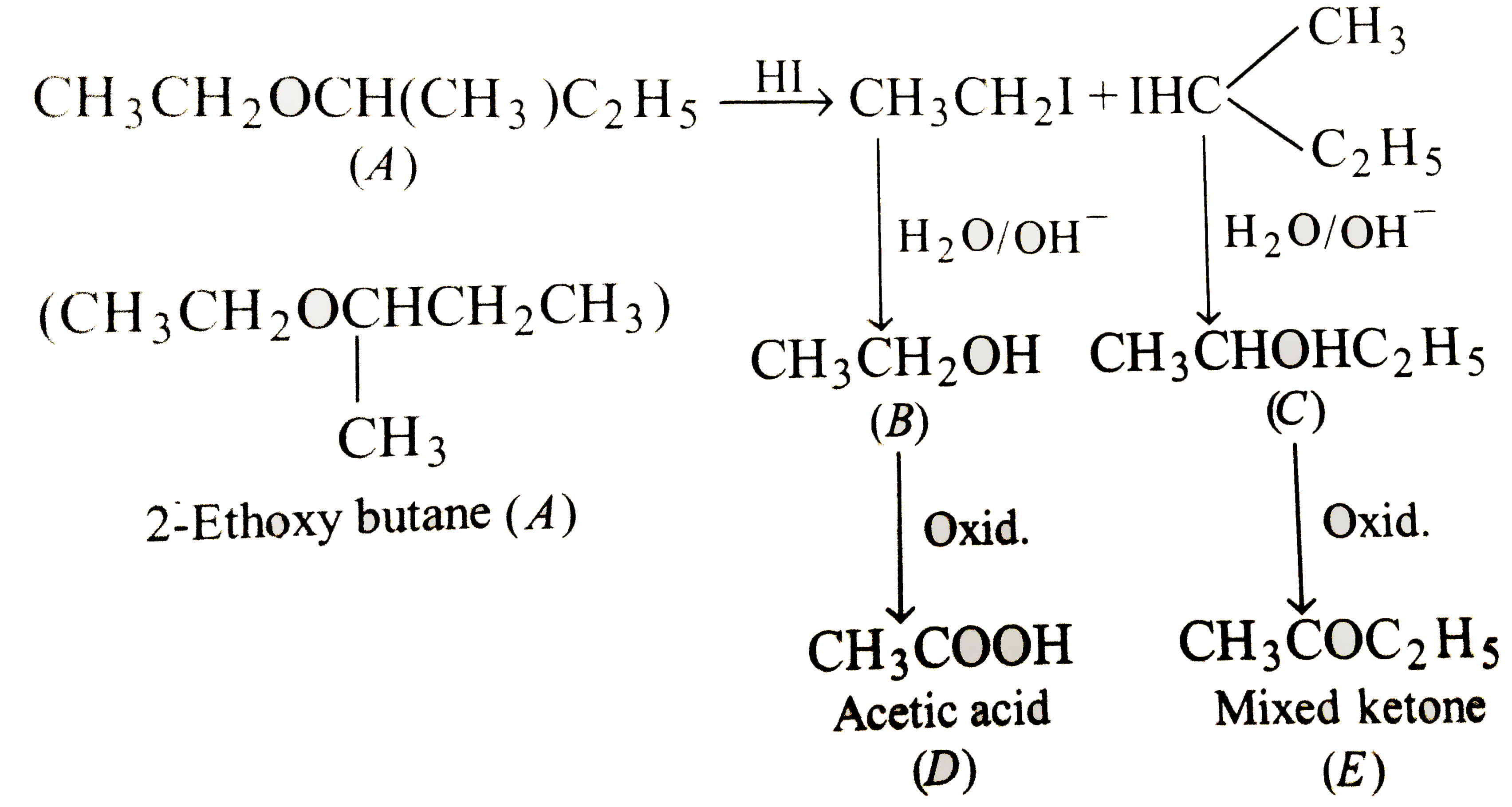

![mole to mole conversion 7.6 [TIMBERLAKE] Chemistry - YouTube mole to mole conversion 7.6 [TIMBERLAKE] Chemistry - YouTube](https://i.ytimg.com/vi/NwtQ9f6tKN8/maxresdefault.jpg)